About
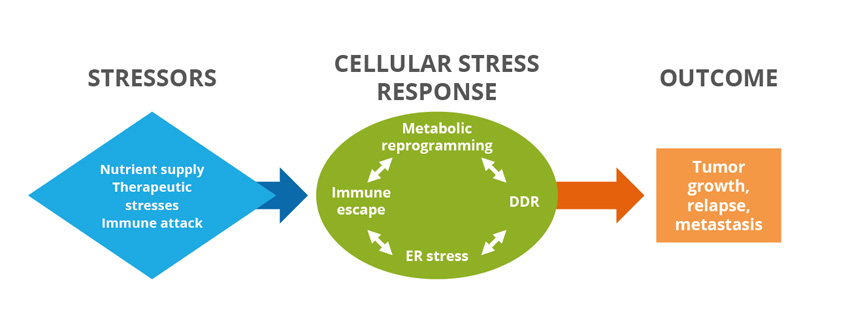
The ASTER team objective is to decipher how malignant cells cope with the inhospitable environment to which they are exposed throughout tumor progression, ranging from microenvironmental alterations to therapeutics. The team focuses on the interconnected adaptive responses to specific environmental stressors, i.e. low nutrition, immune response, and genotoxic agents. The goal of ASTER is to provide an integrated view of these adaptive responses and to identify their vulnerabilities to propose novel therapeutic strategies.
Research projects
First objective
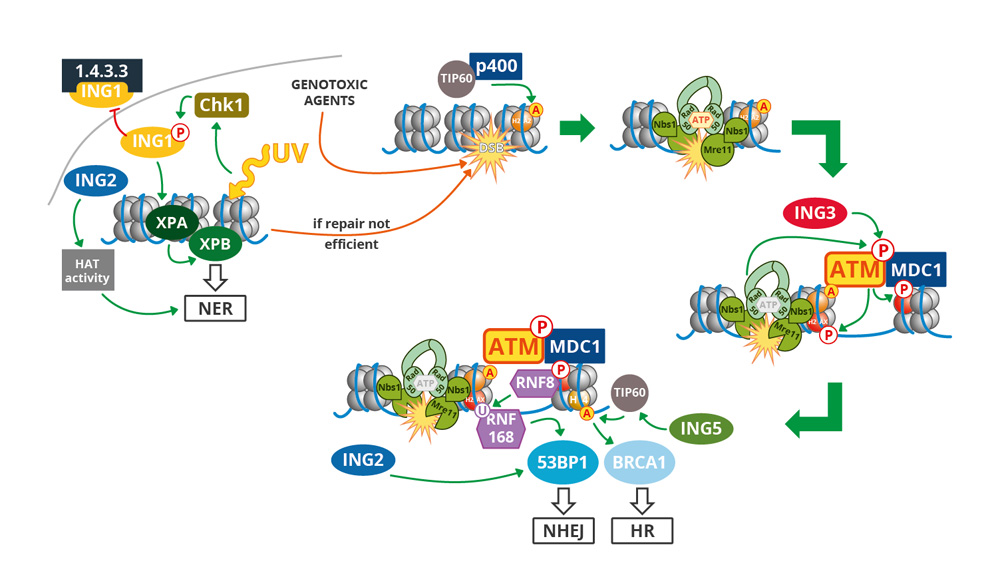
In a first objective, we are investigating the regulations and functions of ING (Inhibitor of Growth) tumor suppressor genes. Our work aims at identifying new tumor-suppressive functions for these proteins (especially ING2 and ING3). We are also interested in the interplay between DDR and glycosylation and DDR impact on inflammation.
Second Objective
Third objective
The third objective is to better characterize the tumor immune microenvironment of Breast tumors with an emphasis on the triple negative subtype (TNBC), one of the most aggressive subtypes of breast cancer, using immunology and high throughput genomic approaches. To this end we established since 2012 a longitudinal biobank of TNBC with the systematic collection of fresh tumors in patients that underwent surgery in CLCC. In line with this objective, we also aim at better deciphering the early anti-tumor immune response in non-clinically invaded lymph nodes in both TNBC and Head and Neck Carcinoma, and at better integrating the tumor microenvironment ecosystem.